ℹ️ This website is strictly for educational and informational purposes. We are not medical professionals and cannot provide dosage advice or medical guidance.
⚠️ Please consult with a qualified healthcare provider before using any substances.
Tirzepatide Storage and Expiration: A Complete Guide
Understanding Tirzepatide Stability
Tirzepatide is a 39-amino acid synthetic peptide that mimics natural incretin hormones, making it exceptionally sensitive to environmental conditions. The peptide’s complex molecular structure is vulnerable to degradation through oxidation, hydrolysis, and thermal denaturation—processes that significantly reduce its therapeutic effectiveness if storage protocols are not followed precisely.
Shelf Life Timeline
Lyophilized (Freeze-Dried) Powder:
When stored in its original freeze-dried form, Tirzepatide maintains excellent stability. At -20°C or colder, lyophilized Tirzepatide remains stable for up to 48 months. Short-term storage at room temperature (15-25°C) is acceptable for only a few days to weeks, though this should be avoided for quality assurance.
Reconstituted Solution:
Once reconstituted with bacteriostatic water, Tirzepatide becomes significantly more vulnerable to degradation. Proper refrigeration extends its usability to 28-30 days when stored at 2-8°C (36-46°F). This is the most conservative and widely recommended timeframe for multi-dose vials, primarily due to sterility concerns after first puncturing the vial.
Expiration Date Example
If you reconstitute Tirzepatide today (November 7, 2025), the recommended expiration date would be approximately December 5-7, 2025 (28-30 days), with an extended window possible until January 7, 2026 (60 days) under optimal conditions.
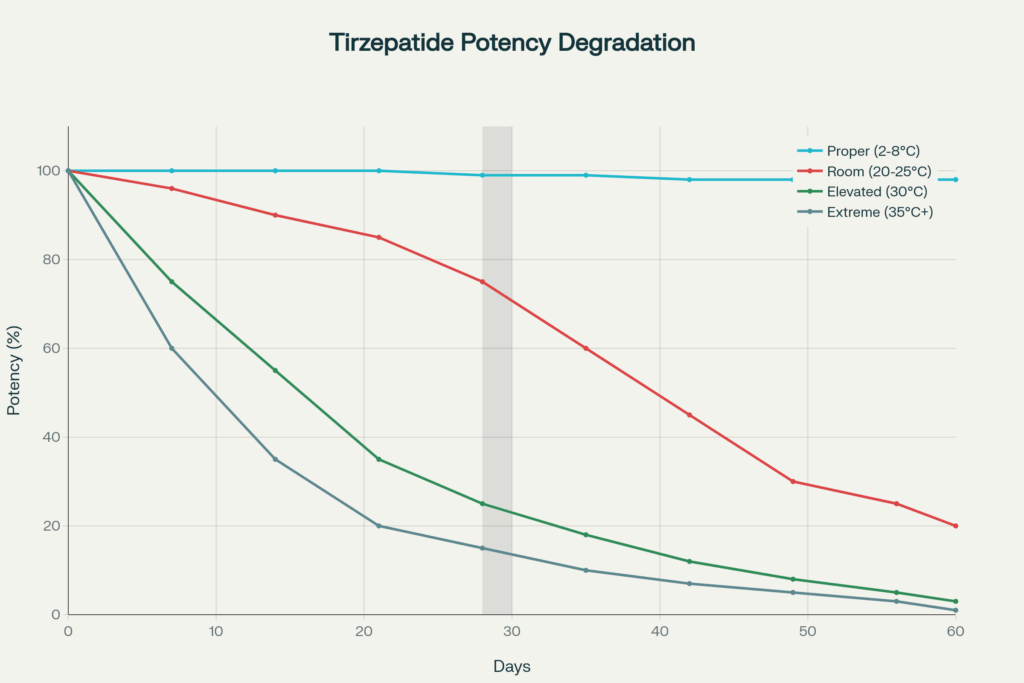
Visual representation of Tirzepatide degradation rates under different storage temperature conditions over time
Temperature’s Critical Role in Degradation
Temperature is the single most significant factor affecting tirzepatide stability. Research demonstrates dramatic differences in degradation rates depending on storage conditions:
Refrigerated Storage (2-8°C):
When properly refrigerated, tirzepatide degradation is minimal. The reconstituted solution remains stable throughout the labeled shelf life with negligible potency loss. Stability studies conducted by manufacturers demonstrate that tirzepatide maintains its therapeutic effectiveness through its expiration date under these conditions.
Room Temperature (20-25°C):
At room temperature, tirzepatide begins degrading gradually but noticeably. Tirzepatide pens and vials can remain at room temperature (below 86°F/30°C) for a maximum of 21 days before degradation accelerates significantly. Beyond this period, the peptide structure begins breaking down at an accelerated rate, reducing potency and potentially forming degradation.
Elevated Temperature (30°C+):
Exposure to temperatures above 30°C (86°F) causes rapid degradation. At these temperatures, tirzepatide effectively expires immediately, regardless of the printed expiration date. Heat exposure of just hours can cause significant structural damage to the peptide, reducing or eliminating its therapeutic efficacy.
Freezing (Below 0°C):
Contrary to common storage assumptions, freezing reconstituted tirzepatide is detrimental. Freeze-thaw cycles cause ice crystal formation that damages the peptide structure through osmotic stress and mechanical disruption. If tirzepatide has been frozen, it must be discarded immediately as the peptide is rendered ineffective and potentially unsafe for use.
Decay Mechanisms and Degradation Pathways
Tirzepatide degrades through multiple chemical mechanisms when storage conditions are compromised:
Oxidation:
Exposure to oxygen initiates oxidative degradation, particularly affecting amino acid residues vulnerable to oxidation (Met, Cys, Trp). This process modifies the peptide’s functional structure, reducing its ability to bind GIP and GLP-1 receptors effectively.
Hydrolysis:
Moisture exposure causes hydrolytic breakdown, where water molecules break peptide bonds. This process shortens the peptide chain or alters its sequence, fundamentally compromising its activity. Humidity above optimal levels accelerates this degradation significantly.
Deamidation:
At elevated temperatures and inappropriate pH levels, asparagine and glutamine residues undergo deamidation—a process where the amide group is converted to a carboxyl group, altering the peptide’s structure and reducing potency.
Aggregation and Fibrillation:
Under stress conditions (agitation, improper pH, temperature fluctuations), tirzepatide molecules can self-assemble into inactive aggregates or fibrils. This is particularly problematic as aggregated peptides are non-efficacious and may pose clinical toxicity risks.
Photodegradation
Light exposure, particularly UV radiation, causes photodegradation of tirzepatide. This process can alter the drug’s chemical composition and reduce therapeutic effects. The peptide’s molecular structure is susceptible to light-induced changes that may form new chemical entities, potentially affecting safety and efficacy profiles.
Practical Storage Guidelines
Essential Storage Requirements:
Store reconstituted tirzepatide in a refrigerator at 2-8°C (36-46°F) in the original vial away from direct light. Use airtight, amber-colored or opaque containers to minimize light exposure and moisture ingress. Never shake the vial—gentle swirling only—as mechanical agitation can cause peptide aggregation and denaturation.
Do Not:
- Freeze reconstituted tirzepatide
- Expose to temperatures above 30°C
- Store in direct sunlight
- Shake vigorously
- Return to refrigerator after room temperature exposure for more than 21 days
- Leave unrefrigerated for extended periods
Container and Sealing:
Reconstituted tirzepatide should be stored in low-binding, airtight vials with tight-fitting caps. The vial should remain tightly sealed when not in use to prevent oxidation and moisture absorption.
Why NovaVitality Ships Lyophilized Powder
The stability advantage is decisive: lyophilized Tirzepatide remains effective for up to 48 months at -20°C, compared to just 28-30 days once reconstituted. Shipping pre-reconstituted solutions would mean customers receive a product already partway through its shelf life, with additional degradation risk during transit from temperature fluctuations.
By shipping lyophilized powder, NovaVitality ensures customers receive a stable, undegraded product that arrives safely without requiring specialized cold-chain logistics. Customers then control their own reconstitution timing, maintaining full potency through the entire 28-30 day window. This approach prioritizes product integrity, regulatory compliance, and customer value—making it the scientifically sound choice for reliable pharmaceutical distribution.




